Cannabis Regulations Published – Licensing Basics

Following Royal Assent of the Cannabis Act (the "Act") on June 21, regulations supporting the implementation of the Act were published on July 11. The regulations include the Cannabis Regulations and the Industrial Hemp Regulations, among others, and they will come into force, together with the Act, on October 17.
The Cannabis Regulations add 218 pages of detail to the basic cannabis legalization framework established by the Act. Though Health Canada implementation policies and guidance, and application forms, remain to be issued, prospective cannabis licensees are now in a position to determine what classes of licence (or what additional classes for current licensed producers) must be obtained to permit their desired cannabis business activities.
As most readers will know, where the desired activity is the retail sale of cannabis for recreational purposes, provincial legislation will dictate what opportunities are available to industry participants. This article focuses on federal licenses to be issued by Health Canada for the following activities:
- Cultivation and processing of cannabis;
- Sale of cannabis (to end users for medical purposes, and amongst industry participants);
- Analytical testing of and research relating to cannabis; and
- Import and export of cannabis.
The article will also address what will happen to licences held by the 112 businesses holding a licence issued under the Access to Cannabis for Medical Purposes Regulations (“ACMPR”) and the much larger group of current ACMPR licence applicants.
Note that references to cannabis in this article do not include industrial hemp. Authorizations and licences for industrial hemp will be discussed in a future newsletter.
CANNABIS CLASSES
To understand what activities are permitted to each class of cannabis licensee it is useful to know what specific forms of cannabis are permitted (and to be aware of the terminology used) under the Act and Cannabis Regulations:
- Five classes of cannabis. The Cannabis Regulations treat different forms of cannabis in different fashion. Although the collective term "cannabis" is used for many purposes in the Act and Cannabis Regulations, certain licensing rules differentiate between the five distinct classes of cannabis and – importantly - no licensee is authorized to sell any cannabis product unless it is one of the authorized classes. Though the list of cannabis classes may expand over time as new permitted classes are introduced, the current authorized classes of cannabis are:
- Dried cannabis;
- Fresh cannabis;
- Cannabis oil;
- Cannabis plants; and
- Cannabis plant seeds.
- Pre-sale cannabis versus packaged cannabis. The five classes of cannabis identified above are further distinguished in the Cannabis Regulations from what are referred to as “cannabis products.” A cannabis product is cannabis of any authorized class that is packaged and labeled for retail sale (but excluding pharmaceutical cannabis drugs).
CANNABIS LICENCES
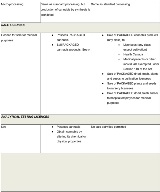
The Cannabis Regulations identify the following six licence categories:
- Cultivation
- Processing
- Analytical testing
- Research
- Sale for medical purposes
- Cannabis drugs
Standard cultivation licences will authorize the growing and harvesting of cannabis, including ancillary activities such as trimming and milling. Micro-cultivation or so-called "craft licences" will authorize the same activities, albeit subject to a 200 square meter canopy limit and lighter security requirements. Nursery cultivation licences will allow for the production of cannabis plants and seeds, but not dried cannabis, fresh cannabis or cannabis oil. Cultivation licences may also permit certain non-retail sale and distribution activities.
Standard processing licences will authorize holders to produce oils, which are not permitted under cultivation licences. Micro-processing licences will authorize similar activities. However, micro-processing licenceholders will be subject to a possession limit of 600 kilograms of dried cannabis (or the equivalent thereof in other classes of cannabis) in a single calendar year. The only exemption to this limit is if the micro-processor also holds a micro-cultivation licence in relation to the same site, and the cannabis held is exclusively from that site. Like cultivation licences, processing licences may permit certain non-retail sale and distribution activities.
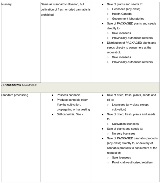
Analytical testing licences authorize laboratories to conduct testing on cannabis as required by the Cannabis Regulations. The Cannabis Regulations require mandatory testing of each batch of cannabis (other than plants or seeds) that will become a retail-ready “cannabis product.” The scope of mandatory testing includes testing for solvent residues, microbial and chemical contaminants that exceed generally accepted thresholds set out in the Food and Drugs Act, and cannabinoid quantities.
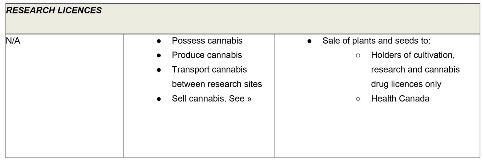
Research licences permit laboratories to conduct more general research relating to cannabis. These licences authorize a narrow range of activities (possession, production, transportation and sale) for research purposes only. As with cultivation and processing licences, holders of research licences may have a right to distribute cannabis to certain other industry participants.
Sale for medical purposes licences are a stand-alone licence category under the Cannabis Regulations. This licence class is distinguished from all other federally-authorized sale and distribution activities (i.e., between industry participants), which are treated as subsidiary authorizations that may be granted to holders of Cultivation, Processing, Research or Sale for Medical Purposes licences. The table below identifies the activities that may be permitted under each class of licence, including permissible non-retail sale activities.
PERMITTED SALE ACTIVITIES
The activities identified as permitted below will not invariably be authorized under each licence of the specified class. Rather, from the activities potentially permitted by the Cannabis Regulations, the applicant will have discretion to determine which are desired and Health Canada will have discretion to determine which will in fact be authorized.
As mentioned, the Cannabis Regulations treat cannabis products prepared for retail sale differently from the pre-sale cannabis of an authorized class (i.e., dried, fresh, oil, plants and seeds). The table below will refer to products as “PACKAGED” when referring to retail sale-ready cannabis products.

Cannabis drug licences under the Cannabis Regulations authorize pharmaceutical drugs containing cannabis for medical purposes. Such drugs will be subject to both the Cannabis Act and the Food and Drugs Act. Furthermore, and unlike the ACMPR, the Regulations contain provisions relating to cannabis drugs for veterinary use. Holders of a cannabis drug licence are authorized to possess cannabis and manufacture or sell and distribute a drug containing cannabis.
IMPORT AND EXPORT
Cannabis may only be imported or exported by licensees for medical or scientific purposes. In addition to holding an appropriate cannabis licence under the Act, licensees must obtain an import or export permit in order to permit such activities for the limited permitted purposes.
TRANSITION OF ACMPR LICENCES AND APPLICATIONS
The Access to Cannabis for Medical Purposes Regulations made under the Controlled Drugs and Substances Act will be repealed on October 17, 2018. However, the ACMPR will be concurrently resurrected as Part 14 of the Cannabis Regulations.
Under the transitional provisions of the Cannabis Regulations, licences issued under the former ACMPR are automatically deemed to be licences under the Act and applications under the ACMPR to be applications for a licence under the Act. The specific sub-class of cannabis licence into which the ACMPR licence will convert will depend on which Cannabis Regulation sub-class captures the authorizations given (or applied for) in the ACMPR licence (or application). The table below summarizes the conversion metric for ACMPR licensees and applicants.
|
ACMPR licence |
Cannabis Act licence |
Possible Cannabis Act licence sub-class |
|
Licence authorizing the production of fresh or dried cannabis, or cannabis plants or seeds
|
Cultivation licence |
Standard cultivation licence |
|
Micro-cultivation licence |
||
|
Nursery licence |
||
|
Licence authorizing the production of cannabis oil or cannabis resin
|
Processing licence |
Standard processing licence |
|
Micro-processing licence |
||
|
Licence authorizing sale of cannabis plants, seeds, fresh or dried cannabis, or cannabis oil to medical users, etc. |
Licence for sale |
Licence for sale for medical purposes |
CRA CANNABIS LICENCES
Cannabis licence applicants should also be aware that any business cultivating, producing or packaging cannabis will be required to be obtain a cannabis licence from Canada Revenue Agency. More information is available on CRA’s website, accessible here: CRA Cannabis Licence.